Laboratory diagnostics: Ileitis (Lawsonia intracellularis)
Assays available:
Gross pathology
-
Evaluates the presence of tissue lesions which can suggest the presence of disease.
-
Location of lesions: terminal ileum (sometimes may include cecum)
-
There are 3 very distinct forms of the disease:
-
Proliferative hemorrhagic enteropathy (acute/peracute)
-
Intestinal mucosal thickening
-
Hemorrhagic content with clots
-
-

Photo 1. Photograph of pig ileum from a peracute ileitis cases showing what appears to be slightly distended intestines with hemorrhagic intestinal content mixed with some partially digested feed content.
Porcine intestinal adenomatosis (chronic uncomplicated)
-
Severe corrugated intestinal thickening
-
Usually no blood
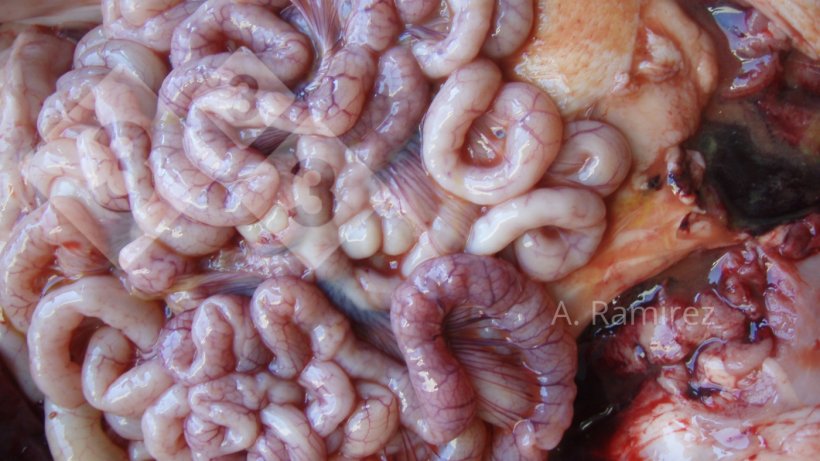
Photo 2. Photo of some slightly corrugated small intestines (due to intestinal wall thickening) suggesting mild to moderately chronic and uncomplicated ileitis. If the intestines we cut open, the thickening and corrugation would become more evident.
Necrotic enteritis (chronic complicated)
-
Severe corrugated intestinal thickening
-
Fibrinonecrotic membrane over thickened intestine
-
Usually no blood
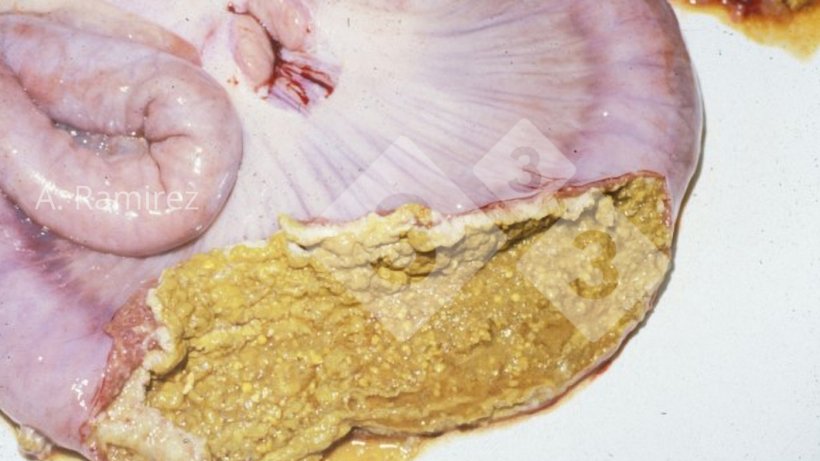
Photo 3. Photograph of pig ileum showing necrotic membrane attached to surface of the intestinal mucosa.
-
Pros:
-
Presumptive diagnosis can be made after gross examination of intestines.
-
Can differentiate the 3 distinct forms of the disease.
-
-
Cons:
-
Lesions not exclusive/diagnostic to ileitis; other diseases can cause similar lesions.
-
Evaluation of thickening can be subjective especially when intestinal contractions can temporarily make the intestinal walls appear thicker than normal.
-
Histopathology
-
Evaluates the presence of tissue lesions (thickening) which can confirm the presence of disease.
-
Sample types: tissue
-
Pros:
-
Histopathology lesions are almost diagnostic
-
Presence of immature crypt epithelial cell proliferation can be confirmed.
-
Minimal or no inflammatory cell infiltration noted.
-
-
Can detect mild versions of the disease
-
-
Cons:
-
Need formalin-fixed intestinal tissues from recently dead animal
-
Occasionally, disease can be segmental especially early in disease
-
Immunohistochemistry (IHC)
-
Detects presence of bacterial antigen
-
Sample types: tissues
-
Pros:
-
Gold standard
-
Detects bacteria at site of lesion (good proof of causation)
-
Can identify low vs moderate vs high amounts of bacteria present
-
-
Cons:
-
Requires significantly more bacteria to be present than PCR
-
Polymerase chain reaction (PCR)
-
Detects presence of specific sequence of bacterial nucleic acid (DNA)
-
Sample types: intestinal tissues, fecal swabs, feces
-
Pros:
-
High sensitivity
-
PCR quantification is associated with presence of lesions
-
Moderate cost
-
Can often do pooling of feces or tissue samples to lower cost while minimizing loss of sensitivity (especially regarding clinical relevance).
-
-
-
Cons:
-
High sensitivity – confirms presence of bacteria but not disease
-
Unable to differentiate bacteria from live vaccine vs. wildtype infection
-
Enzyme-linked immunosorbent assay (ELISA)
-
Detects presence of antibodies
-
Sample types: serum
-
Pros:
-
Animals remain positive for several weeks
-
Can be used in chronic cases
-
Higher diagnostic sensitivity than indirect fluorescent antibody (IFA) assay
-
-
Cons:
-
Specific antibodies detected and the timing of detection may vary slightly between the different commercial kits available.
-
Takes 2 to 4 weeks depending on bacterial load for animals to become seropositive.
-
Unable to differentiate bacteria from live vaccine vs. wildtype infection.
-
Indirect fluorescent antibody (IFA)
-
Detects presence of antibodies
-
Sample types: serum
-
Pros:
-
Animals remain positive for several weeks
-
Can be used in chronic cases
-
-
Cons:
-
Lower diagnostic sensitivity than ELISA
-
Not feasible for large number of samples
-
Takes 2 to 4 weeks depending on bacterial load for animals to become seropositive.
-
Unable to differentiate bacteria from live vaccine vs. wildtype infection
-
Reliability is highly dependent on technician skills
-
Result interpretation:
Gross pathology
-
Positive: Often presumptive diagnosis is sufficient
-
Negative: No gross intestinal lesions
Histopathology
-
Positive: Strong association between thickening and disease
-
Negative: No intestinal lesions
IHC
-
Positive: Bacteria is present at site of lesion
-
Negative: Negative or infection is too early (slow growing bacteria) or too late after infection.
PCR
-
Positive:
-
Ct <20 strong link to intestinal lesions (IHC) and disease
-
Ct>30 indicate organism present but not associated with intestinal lesions (IHC negative) and thus not likely to have an economic impact at that time.
-
-
Negative: Negative or bacteria could have been missed if testing occurs too early (slow growing bacteria) or too late after infection.
ELISA
-
Positive: Past exposure (> 2-4 weeks) to vaccine or wildtype bacteria
-
Negative:
-
Negative to vaccine or wildtype bacteria
-
Infection too early to detect (slow growing bacteria)
-
Antibiotic use may suppress bacterial growth and lower immune response.
-
IFA
-
Positive: Past exposure (> 2-4 weeks) to vaccine or wildtype bacteria
-
Negative:
-
Negative to vaccine or wildtype bacteria
-
Infection too early to detect (slow growing bacteria)
-
Antibiotic use may suppress bacterial growth and lower immune response.
-
Scenarios
Growing pigs with scours (acute or chronic):
-
Collect fecal samples from 10 or more untreated scouring pigs and submit for PCR testing in pools of 5.
-
Necropsy 1-3 recently dead pigs or euthanize scouring pigs. Grossly evaluate terminal ileum for thickening and collect ileum sample and place in formalin solution for histopathology and IHC evaluation.
Finishing pigs that are not growing (chronic):
-
Necropsy 1-3 recently dead pigs or euthanize scouring pigs. Grossly evaluate terminal ileum for thickening and collect ileum sample and place in formalin solution for histopathology and IHC evaluation.
Determining timing of exposure:
-
Collect samples from 10-15 pigs at 8, 11, 14, and 17 weeks of age
-
Two approaches to collecting:
-
Cross-section – collect from different age groups at one time (quicker to obtain results).
-
Longitudinal – collect from same pigs over time (results are more accurate).
-
-
Serum samples test via ELISA (preferred) or IFA.
-
Fecal samples test via PCR in pools of 5.
-







